Background: Varnimcabtagene autoleucel (IMN-003A) is an anti-CD19 CAR-T cell immunotherapy for patients with relapsed refractory B cell malignancies (RR BCM) in the Phase 2 IMAGINE study. Some patients (pts) do not respond or relapse early after CD19 directed CAR-T cell therapy. This abstract reviews the factors associated with disease relapse to help improve patient outcomes and development of novel strategies.
Methods: Patients (pts) aged 3 to 45 years (B-ALL) and ≥ 18 years (B-NHL) with RR BCM were eligible for IMAGINE study if they had measurable disease, as assessed by lymphoid blasts (B-ALL) or metabolic tumour bulk (B-NHL), received ≥1 prior regimen, refractory to the last line of treatment with good performance status (ECOG 0 to 1).
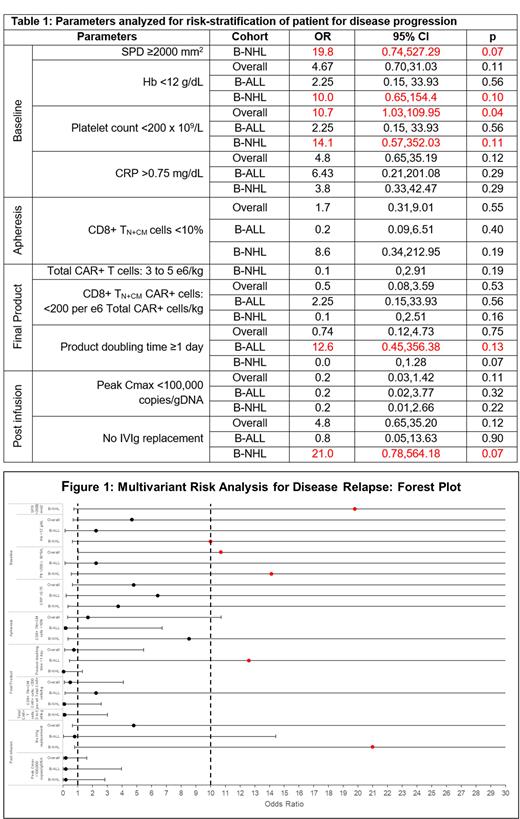
Multiple characteristics for disease progression were reviewed (n=36) and risk stratified as: 1) Baseline parameters (age, lines of treatment, bridging therapy, cytogenetics, blasts, total metabolic tumour volume (TMTV), sum of perpendicular diameter (SPD), refractory, relapse within 6 months, laboratory parameters - haemoglobin, neutrophil count, lymphocyte count, platelet count, CRP, LDH, ferritin, baseline interleukin-6 (IL-6), baseline total T cells, CD4, and CD8 subsets in peripheral blood); 2) Apheresis parameters (total T cells, naïve / central memory in CD4 and CD8 subsets); 3) Final Product characteristics [total CAR+ cells, naïve / central memory in CD4 and CD8 subsets, CD8 (naïve & central memory subsets / tumour burden (TMTV and SPD), product doubling time] and 4) Post Infusion factors (CAR peak & persistence, B cell aplasia, hypogammaglobulinema, infections and intravenous immunoglobulin (IVIg) replacement).
These parameters were analyzed using chi-square and appropriate multivariant odds ratio (OR) to examine the association with disease relapse.
Results: At data cut-off, of 25 pts enrolled (median age 31 yrs, range 3 - 66) with RR BCM (n=13 B-ALL; n=12 B-NHL), 24 pts received var-cel (1 withdrawal). Median follow-up after IMN-003A administration was 205 days (range 12 - 434).
Overall response rate (ORR) was 91.7% (22/24) at D+28 (B-ALL 91.7%; B-NHL 91.7%) and 80.9% (17/21) at D+90 (B-ALL 80%; B-NHL 81.8%). Median progression free survival (PFS, range 12-NR), duration of response (DOR, range 0-NR) and overall survival (OS) were not reached (range 12-NR). Treatment related mortality was 4.2% (n=1/24); 3 pts died of disease progression.
Nine pts had relapsed disease (B-ALL 4; B-NHL 5; overall 37.5%; CD19+ 7 pts; CD19- 2 pts; B-ALL: CD20 variable; CD22+ n=4/4; B-NHL PDL1 n=1/5) with median PFS of 178 days (range 28-319). For relapsed pts, median duration of B cell aplasia after primary infusion was 270 days (range 70-NR); var-cel was detected in 1 pt (n=1/4) at relapse and hypogammaglobulinema (IgG <4g/L) was seen in 4 pts.
For the 36 multiple parameters studied, age, high risk cytogenetics / genomic aberrations, refractoriness / early relapse <6m, blasts burden in B-ALL, absolute lymphocyte count, inflammatory markers (LDH, ferritin) and baseline T cell subsets did not show association with disease relapse.
Ten variables showing some association with disease progression on univariant analysis were: SPD, baseline haemoglobin, platelets, CRP, Apheresis CD8+ T N+CM, Final Product doubling time, CAR-T dosage (B-NHL), CD8 CAR+ T N+CM (weight & dose adjusted), Post Infusion Cmax and IVIg replacement.
Multivariant analysis of these parameters showed some statistical significance for following variables: Baseline Platelet count <200 x 10 9/L (overall); Final Product doubling time ≥1 day (B-ALL); baseline SPD ≥2000 mm 2, Hb <12 g/dL, Platelet count <200 x 10 9/L and post infusion IVIg replacement (B-NHL), Table1 & Figure 1. Of these variables, statistically significant association was seen with Final Product doubling time ≥1 day (B-ALL) and baseline SPD ≥2000 mm 2 with Hb <12 g/dL and Platelet count <200 x 10 9/L (B-NHL). The impact of IVIg replacement on efficacy needs further study.
Conclusions: In the IMAGINE study, with ORR 80.9% at primary endpoint and median PFS not reached, relapse was seen in 37.5% pts with median PFS of 178 days. Multivariant analysis has identified factors which may predict disease relapse. Further research is needed to identify strategies such as reinfusion or CAR-T targeting a different antigen to achieve cure.
Disclosures
Raju:Immuneel Therapeutics Private Limited: Current Employment. Arasu:Immuneel Therapeutics Private Limited: Current Employment. Elluru:Immuneel Therapeutics Private Limited: Current Employment. Akheel:Immuneel Therapeutics Private Limited: Current Employment. Kamat:Immuneel Therapeutics Private Limited: Current Employment.